
Xiao-Ping Zhou, Zhengtao Xu,* Jun He, Matthias Zeller, Allen D. Hunter, Rodolphe Clérac, Corine Mathonière, Stephen Sin-Yin Chui, and Chi-Ming Che
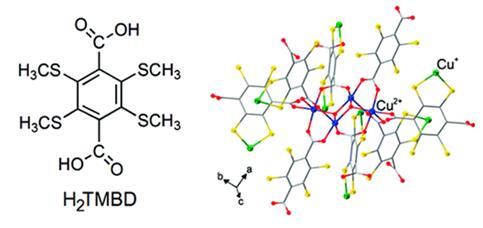
Abstract: This paper aims to illustrate the rich potential of the thioether-carboxyl combination in generating coordination networks with tunable and interesting structural features. By simply varying the ratio between Cu(NO3)2 and the bifunctional ligand tetrakis(methylthio)benzenedicarboxylic acid (TMBD) as the reactants, three coordination networks can be hydrothermally synthesized in substantial yields, which present a distinct evolution with regard to metal-ligand interactions. Specifically, Cu(TMBD)0.5(H2TMBD)0.5 3 H2TMBD (1) was obtained with a relatively small (1:1) Cu(NO3)2/TMBD ratio, and crystallizes as an one-dimensional (1D) coordination assembly based on Cu(I)-thioether interactions, which is integrated by hydrogenbonding to additional H2TMBD molecules to form a three-dimensional (3D) composite network with all the carboxylic acid and carboxylate groups remaining uncoordinated to the metal ions. A medium (1.25:1) Cu(NO3)2/TMBD ratio leads to compound Cu2TMBD, in which Cu(I) ions simultaneously bond to the carboxylate and thioether groups, while an even higher (2.4:1) Cu(NO3)2/TMBD ratio produced a mixed-cation compound CuII 2OHCuI(TMBD)2 3 2H2O (2), in which the carboxylic groups are bonded to (cupric) CuII ions, and the thioether groups to CuI. Despite the lack of open channels in 2, crystallites of this compound exhibit a distinct and selective absorption of NH3, with a concomitant color change fromgreen to blue, indicating substantial network flexibility and dynamics with regards to gas transport.
文章链接:![]() ic101501p.pdf
ic101501p.pdf




